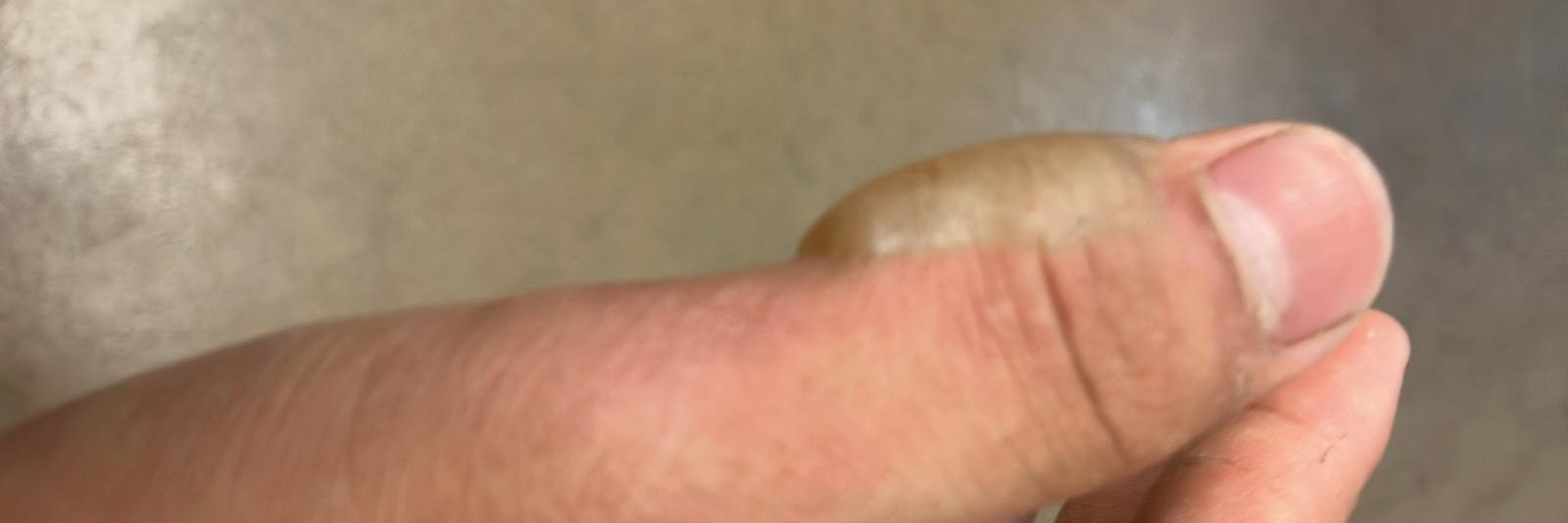
The other day, as a result of playing with gasoline, I got some second-degree burns.
Eight years ago, in preparation for a backpacking trip to Sword Lake, my dad bought a 1-gallon can of Coleman Fuel (a petroleum distillate similar to gasoline but without the engine-performance-enhancing additives), to fuel his Coleman 442 pressure stove. After using it for a few camping trips and to melt zinc, the fuel was finally finished as I continued my metal-casting project over this summer break.
Of course, the fuel can wasn’t exactly empty. I did my best to pour out the remaining fuel, but there was always a bit left in the can. Not wishing to cause a fire at the recycling center, I made the rather stupid decision to get rid of the remaining fuel by burning it out.
As you learned in elementary school, fire requires fuel, an oxidizer, and heat. Heat is usually produced by the combustion reaction itself, making it self-sustaining so long as there is fuel and oxidizer available, but if heat isn’t generated fast enough (or if it’s removed too quickly), then the fire goes out. Thus, even if fuel and oxidizer are present, not enough of either will cause a reaction too slow to be self-sustaining. In other words, you need a certain range of fuel-oxidizer ratios for a mixture to be flammable. In the case of gasoline (similar to Coleman Fuel and more widely studied), this range is actually rather narrow: between 1% and 7.6% gasoline vapor by volume, with an ideal percentage of 6.4%.
With perhaps 30 mL of liquid fuel left in the can, I used the spark mechanism of an empty BIC lighter with the hood (the metal shield that covers the nozzle) removed to light the gasoline vapor coming off the mouth of the can, making a small flame at the opening. The vapor inside the can did not burn because it was too concentrated (i.e. the mixture was too rich), while immediately above the opening, its concentration was just right. (And farther away from the opening, its concentration was too small, or it was too lean.) Squeezing the sides of the can pushed a large amount of fuel vapor out instantly, causing a small fireball. As the puddle of fuel remaining at the bottom of the can decreased in size, so did the rate of gasoline vapor production, which caused the flame to get smaller and smaller until it finally went out.
After leaving the fuel can un-lit for a few minutes, I tried to light it again with the BIC lighter. Imagine my dismay when a column of fire immediately rushed out of the opening and caused second-degree burns on my thumb and the base of my middle finger.
What happened was that after leaving the can open for some time with reduced vapor production inside of it, some air was able to get in, allowing the air-fuel mixture in the can to enter the flammability range. (With a bigger puddle of fuel and more vapor production, the vapor leaving the can quickly pushed out most of the air.) When lit with a spark, the mixture all burned at once, producing rapidly expanding hot gas and flames that rushed out of the opening, burning my hand.
The point I’m trying to make by writing this blog post is not that gasoline and fire are dangerous in all circumstances. It’s that you should take the time to understand the risks associated with them so you can use it (and play with it) safely.
UPDATE 2025-08-29: The fine folks at the Coleman company appear to have read my blog post, because their latest fuel-can design includes a flame suppressor at the spout!